By Ro Stastny
September 2, 2025
Chemical engineering student Nuo Xu (Ph.D. ‘25) has led extensive research in the Nance Lab formulating a therapeutic nanoparticle shown to effectively reduce neuroinflammation resulting from fetal growth restriction in newborn piglets. Her success is paving the way for future clinical trials providing the same treatment to human infants.
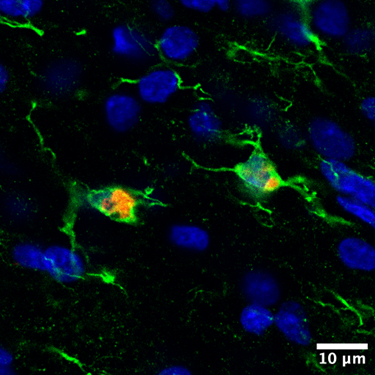
An image showing the localization of the therapy-loaded nanoparticle (colored in red) inside newborn brain cells (green). The blue areas are cell nuclei. Photo provided by the Nance Lab.
In humans, fetal growth restriction (FGR) affects up to ten percent of all pregnancies in developed countries and is a leading cause of perinatal complications and mortality. Life-long consequences of FGR range from learning and behavioral disabilities to cerebral palsy. Formulating targeted treatments is essential to mitigating the long-term impact of newborn brain injuries resulting from FGR.
While existing research on brain therapeutics has shown positive outcomes, developing and scaling these therapies presents several challenges. Curcumin, a molecule with a potent anti-inflammatory response, has been successfully tested and shown to reduce inflammation and heal brain injuries in mice and rats. Because curcumin is not soluble in water, it is not useful to cells in the body on its own. In order to administer it as a treatment, curcumin must be loaded into a drug carrier like a nanoparticle, so it can travel through the body to reach the targeted cells. However, this process could result in a dramatic loss of the molecule's potency and ultimate effectiveness in treating the injury. The developed nanoparticle acting as the carrier in this instance requires careful control of the formulation to maximize the amount of the drug contained in the particle, while also keeping the drug active and intact.
Testing therapies using a pig model
Initial research and experimentation of these kinds of therapeutics is most frequently conducted on FGR mice and rats weighing around 30 grams. Animals of this size are not comparable to a FGR infant weighing closer to two kilograms. Xu collaborated with a clinical lab at the University of Queensland in Australia to administer and test therapeutics for FGR brain injuries of newborn pigs, a model much closer in scale to that of a human infant. The FGR newborn pigs weigh around one kilogram, and show brain injuries very similar to humans as a result of lack of sufficient nutrients during gestation.
Shelf-stable for shipping
Access to this pig model is ideal because the brain injury occurs the same way as it does in humans, but it also presents an additional challenge. The formulated nanoparticles must be highly shelf-stable for several days or weeks during and after shipment to the lab in Australia to be administered. Xu experimented with different methods to prolong the shelf life of the nanoparticles, such as developing a freeze-dried solid powder form as opposed to the unstable liquid form. Freeze drying improved the shelf stability of the nanoparticle and preserved the activity of the drug.
Results and next steps

In June Nuo Xu (left) presented her Ph.D. defense titled "Pharmacokinetics and efficacy of a tunable curcumin-loaded polymeric nanoparticle for neonatal neuroprotection." Photo provided by Elizabeth Nance (right).
So far, intranasal administration in the FGR piglets has shown that over 15% of an administered drug-loaded nanoparticle solution accumulates in injured regions and specific cells in the brain, which is considered successful. The uptake in the brain compared to intravenous administration was five times higher, suggesting that intranasal delivery of this therapy in FGR infants may be similarly successful in the future.
The Nance Lab will continue to use the pig model at the University of Queensland to further establish that this therapy and dose can reduce brain injury and improve health of the FGR piglets, setting the stage for a clinical trial administering the same treatment in human infants.
Nuo Xu presented this research in her Ph.D. defense in June 2025, and is currently making moves to begin her career the pharmaceutical industry.