By Jeremiah Wilhelm
April 21, 2025
Researchers from the University of Washington Department of Chemical Engineering have developed better ways to isolate cells from the brain's protective barrier, creating a roadmap for future scientists to follow. The field of blood-brain barrier (BBB) research represents a critical intersection of neuroscience, engineering and medicine, with significant implications for treating neurological diseases.
Sydney Floryanzia, a chemical engineering Ph.D. candidate in the Nance Lab, conducted this study. Her research demonstrates how standardized isolation methods for primary rat neurovascular cells can address persistent challenges associated with obtaining neurovascular cells in sufficient quantities for experimental studies. These methods not only improve cell yield but also document important morphological benchmarks over time that other researchers can use to evaluate cell isolation effectiveness and design future experiments.
“Our initial goal was to get neurovascular cells that we could use for disease modeling and particle uptake studies. Along the way, we found it was very difficult to obtain these cells because the protocols were so obscure. With this set of methods, we now have relatively pure, isolated astrocytes, pericytes and endothelial cells, enabling us to ask new questions in relation to both two-dimensional and three-dimensional culture systems.”
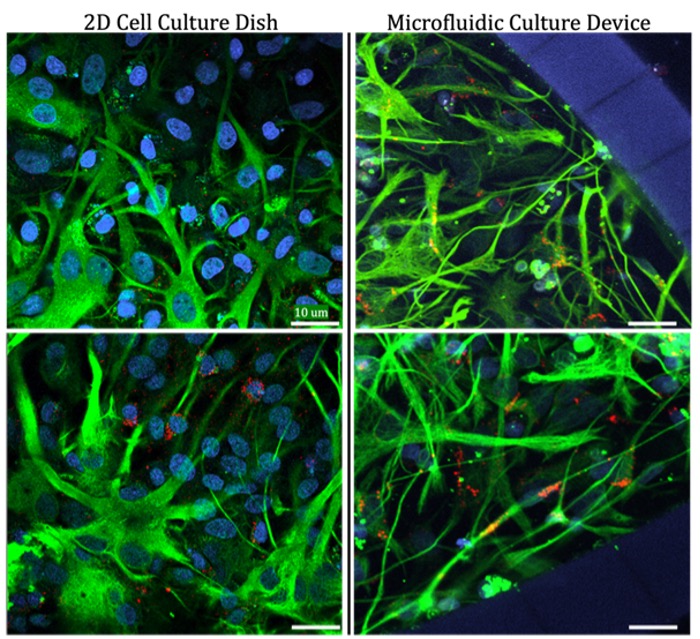
Astrocyte morphology (form or structural) differences based on culture environment: This finding illustrates the ability to modulate primary astrocytes based on culture conditions, as well as the suitability for these cells and microfluidic culture devices for use in disease modeling and therapeutic screening. Image provided by the Nance Lab.
Primary cells isolated from brain tissue offer unique advantages for BBB modeling. However, inconsistent isolation protocols, poor documentation of expected morphological changes and insufficient troubleshooting resources have made it difficult to pin down standardized methods for this type of cell isolation. The primary cells harvested in the Nance Lab include astrocytes, star-shaped support cells in the central nervous system that maintain brain homeostasis; pericytes, contractile cells that wrap around small blood vessels, regulating blood flow, vascular permeability and stability; and endothelial cells, specialized cells that line the interior surface of blood vessels. Together, these cells form a selective barrier between the vessel and surrounding tissues to permit nutrients while protecting the brain against potential toxins in the blood.
While numerous protocols for isolating astrocytes, pericytes and endothelial cells exist, they often lack detailed guidance on critical methodological steps and cell development during culturing. Floryanzia noted that these inconsistencies specifically impact reproducibility and contribute to variable outcomes obtained in BBB model testing.
“We created a consolidated, clear set of protocols with visuals and benchmarks. Now, anyone can easily adapt these cell isolation procedures into their workflow,” said Floryanzia.
The researchers optimized several critical processes that minimized cell trauma. These refinements resulted in higher yield and viability by reducing debris and non-attaching cell death in culture vessels.
A significant contribution of this work is the establishment of clear visual guidelines to both the methodological steps themselves and to changes in the physical structure for each cell type throughout the isolation and culture process. The researchers monitored neurovascular cell growth for more than two weeks in vitro, evaluating cell attachment, maturation and viability. Using phase contrast microscopy, they documented initial cell plating, attachment and daily growth patterns. For mixed glial cultures, they identified distinct morphological shifts at three, five, 10 and 12 days in vitro. Cell identities were confirmed and characterized according to progressive structural changes as witnessed under confocal microscopy and through immunostaining techniques. This morphological documentation provides researchers with visual references to evaluate their cell isolation effectiveness, addressing a critical gap in the field's methodological understanding.
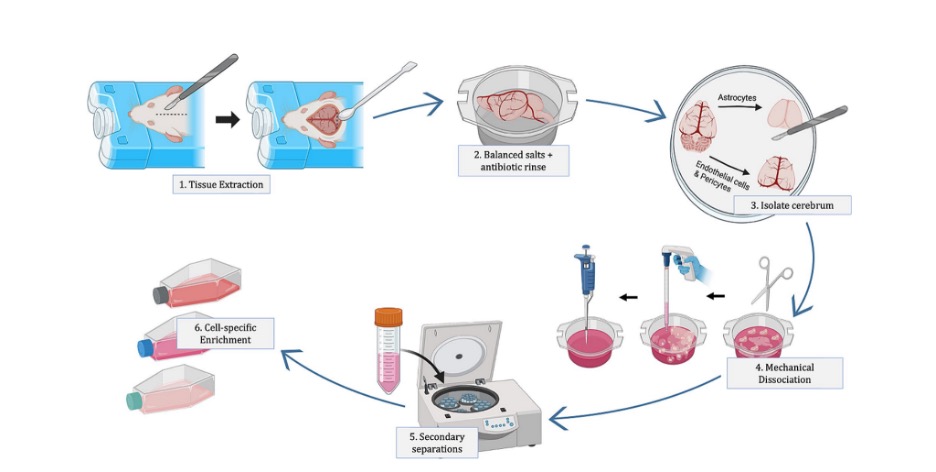
General overview of primary neurovascular cell isolation. Image provided by the Nance Lab.
The implications of this standardized methodology extend well beyond technique improvement. As a selective barrier, the BBB can present significant challenges in developing medicines to treat brain diseases. By increasing primary cell availability, researchers can more effectively develop in vitro BBB models for therapeutic screening and pathology research. The ability to isolate and identify a variety of structural cells allows for investigations into cell-specific therapeutic associations and cross-talk during disease progression. As neurological disorders from Alzheimer's to multiple sclerosis increasingly implicate multiple cell types, including the cells of the BBB, these optimized protocols establish a foundation for more accurate disease modeling and potentially pave the way for developing more effective therapeutic strategies.