By Ro Stastny
October 11, 2023
By using colored light, UW researchers can now manipulate biomaterials deeper inside native tissue than ever before.

A discovery recently published in Nature Communications could revolutionize in vivo medical treatments like controlled drug delivery or regenerative tissue engineering.
Led by Teresa Rapp, former Washington Research Foundation Postdoctoral Fellow in the DeForest Lab, the research identified three crosslinkers — molecules that bind materials together — that degrade rapidly after exposure to visible light. When used in sequence on multilayered hydrogels dyed with patterns corresponding to the different crosslinkers, low-energy red, green and blue lights ultimately degraded crosslinker regions based on the colored light source.
Researchers showcased the results by using red and yellow dyed regions within a collective gel, including a sample bearing the university's signature "W.” The image shows how the gel changes and degrades after exposure to each colored light source.
Going deeper
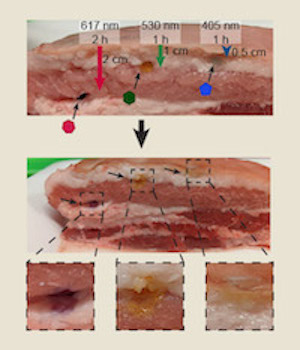
Prior to this research, photodegradable gels used in regenerative medicine have been engineered to respond almost exclusively to UV light. However, UV light is not capable of penetrating tissue depths needed for photocontrolled drug delivery. Since visible light is able to penetrate much deeper into native tissue, these new materials can be optically modulated at previously inaccessible depths.
To demonstrate that these new materials could degrade deep within real tissue, each crosslinker hydrogel was transplanted inside skin-on pork belly. Findings highlighted that the gels could degrade as deep as two centimeters into the skin, a powerful first step toward using these systems for in vivo needleless drug delivery.
Testing cytocompatibility
After confirming that the crosslinkers showed wavelength-specific response when placed inside tissue layers, the next step was to determine the viability of cells that were encapsulated in a crosslinked hydrogel and then released after light degraded that gel.
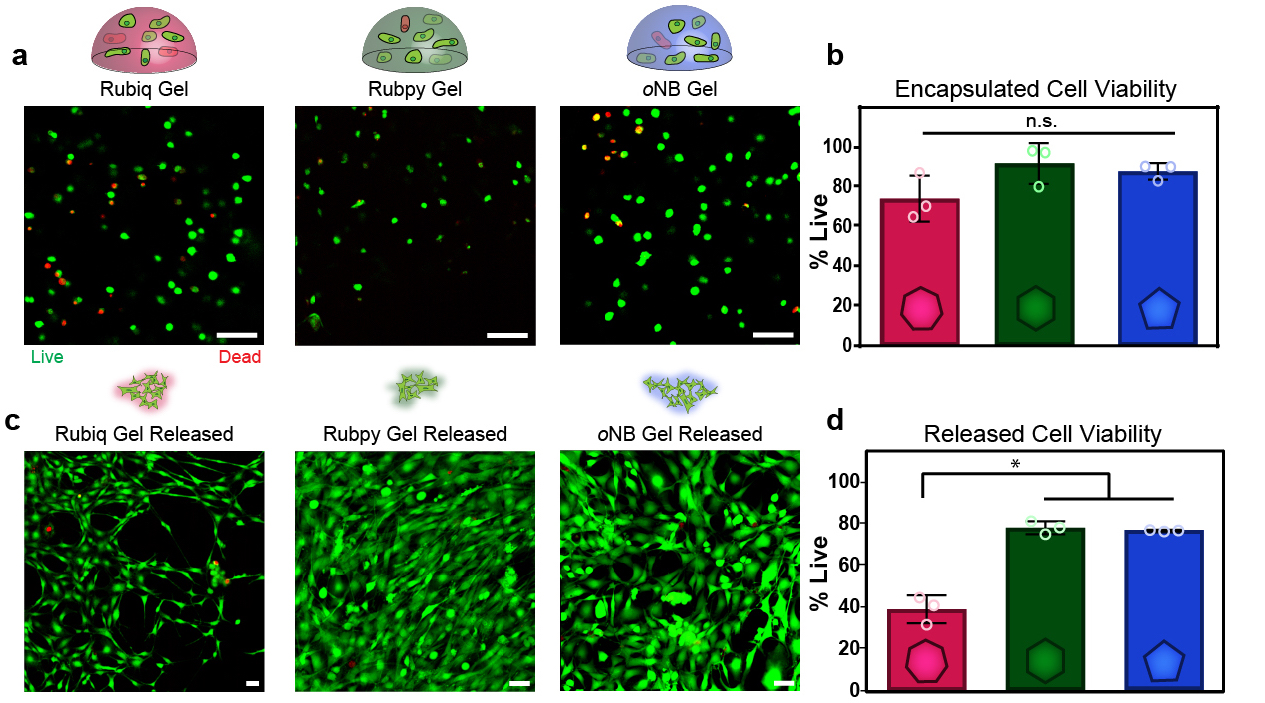
The researchers took stromal cells derived from human bone marrow and placed them within the crosslinked gels. After light exposure "released" the cells from the degraded gel, they measured the level of remaining living cells. Though room for improvement exists for the red light-sensitive system, a significant number of living cells were recovered from each of the material platforms that highlights their overall biocompatibility.
The future of drug delivery
What does this mean for the future of regenerative medicine?
“We’re really interested in using these systems for engineering complex functional tissues and for controlled therapeutic delivery,” says Cole DeForest, professor of chemical engineering and bioengineering and co-author of the research, “particularly for staged and well-dosed needleless drug delivery.”
The research group is using these materials to create engineered microvasculature and improve high-throughput drug screening. Their hope is that these approaches will expand the possibilities of regenerative solutions in medicine.